Statements Correctly Explain a Difference Between Ionic and Covalent Bonds
A Elements in Group 1A are more likely to form a covalent bonds than ionic bonds with other atoms B The difference between electronegativities is greater for atoms in covalent bonds than for atoms in ionic bonds C More electrons are transferred to form. Covalent bonding takes place when the atoms occurs because the atoms in the compound have a similar ability to gain and lose ions.
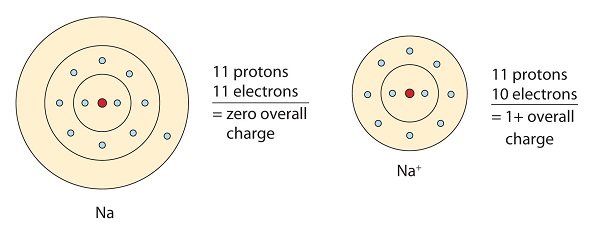
1 3 Ionic And Covalent Bonds Chemistry Libretexts
Materials with covalent bonds are not ductile.
. - In an ionic bond an electron is transferred from one atom to another. The question contains content related to Chemistry and Science. Materials with covalent bonds are not malleable.
The ionic bond is the electrostatic force of attraction between two oppositely. A covalent bond will gain up to 8 electrons to form a bond and an ionic bond will give away up to 8 electrons to form a bond. For the main-group elements Lewis symbols provide information about the ___________ electrons involved in bonding.
- Ionic compounds dissociate into ions in. View solution The electrovalent linkage is present in. 2 on a question.
Difference between Ionic and Covalent Bond. Youll also see these as a gas or liquid rather than a solid like ionic bonds. In relation to each other covalent bonds are the strongest followed by ionic hydrogen bond Dipole-Dipole Interactions and Van der Waals forces Dispersion Forces.
Ionic is losing and gaining electrons Covalent is sharing the same electrons Ionic - One atom loses and electron the other gains one and two oppositely charged ions are produced which are attracted to each other. Materials with ionic bonds are not ductile. Afterwards the atoms are becoming ions and they are separated.
Covalent - A shared pair of electrons resulting in both atoms having full outer shells. In a covalent bond electrons are shared. In an ionic bond one partner accepts electrons from the other.
The main difference between covalent and ionic bonds is that ionic bonds occur between two species which are electrostatically attracted towards each other whereas covalent bonds occur covalently through the sharing of electrons between their outer shells. The more you know. An ionic bond is a chemical bond between two dissimilar ie.
A metal and a non-metal atoms in which one atom gives up an electron to another. Ionic and covalent bonds are the major two types of chemical bonds that exist in compounds. The reaction components of covalent bonds are electrically neutral whereas for ionic bonds they are both.
Since its upload it has received 362 views. What is the fundamental difference between covalent and ionic bonding. In general metallic elements tend to form ionic bonds and non-metallic elements tend to form covalent.
Since covalent bonds are just sharing their stuff they arent as solid of a bond as ionic bonds. In covalent bonds atoms share electrons whereas in ionic bonds atoms transfer electrons. Covalent bonds tend to be less polar than ionic bonds.
How does the formation of cationanions in the bond. Explain the differences between ionic and covalent bonds by answering the following questions. These bonds are the strongest out of the list.
In a covalent bond the two atoms are attached one another with their common electrons. Given below are the points which differentiate among the three types of strong or primary bonds. 8 rows An ionic bond essentially donates an electron to the other atom participating in the bond.
View solution The compound which contains both ionic and covalent bonds is. Covalent bonds can be said when there is the strong electrostatic force of attractions between two positively charged nuclei and the shared pair of electrons. 7Which statement comparing ionic and covalent bonds is correct.
Like a result a pure covalent bond lacks no ionic property. Which bond ionic or covalent involves cations and anions. Materials with metallic bonds are malleable.
They can move independently from one another. Materials with ionic bonds are not malleable. With two suitable examples explain the difference between an ionic and covalent bond.
They attract themselves but they are not necessarily in contact. So ionic bonds may form between metals and nonmetals while covalent bonds form between two nonmetals. One pure covalent bond is formed when two atoms with the same electronegativities come together.
But while covalent bonds might be a little weaker than ionic bonds they can form between the same elements. Begingroup In an ionic bond one atom has lost one electron given to the second. A covalent bond is another strong chemical bond.
It takes place been similar atoms ie. Ionic compounds are actually an ion pair held. The answer is D Ionic bonds are formed by loosing an electron s and covalent bonds are formed by sharing of electron s.
Select all the statements that correctly describe the consequences of electronegativity differences between the nonmetal atoms forming the covalent bond. When two non-metal atoms share a pair of electrons a covalent bond is formed. Key Differences Between Covalent Metallic and Ionic Bonds.
These are referred to as intramolecular bonds whilst the rest are referred to as intermolecular forces. Ionic bonds involve a transfer of electrons from one atom to another. The electrons involved are located in the atoms outer shells.
The statement that aptly captures the comparison between ionic bonds and covalent bonds is. Which statement identifies a difference between an ionic bond and a covalent bond. In covalent bonding both partners end up with filled outer electron shells.
9 rows Ionic bond. Ionic bonds can also be dissolved in water and other types of polar solvents. On StudySoup on 5312017.
In ionic bonding one partner does and the other does not. How do ionic bonds differ from covalent bonds when it comes to what happens to the electrons of the atoms involved in each type of bond. Covalent bonds are formed when a pair of electrons are shared between two atoms in a bond.
The difference between ionic and covalent bond is that ionic bonds occur between atoms having very different electronegativities whereas covalent bonds occur between atoms with similar or very low electronegativity differences. This 39 words question was answered by Heather L. View solution Which of the following compounds contains both ionic and covalent bonds.
In a covalent bond the partners share a pair of electrons.
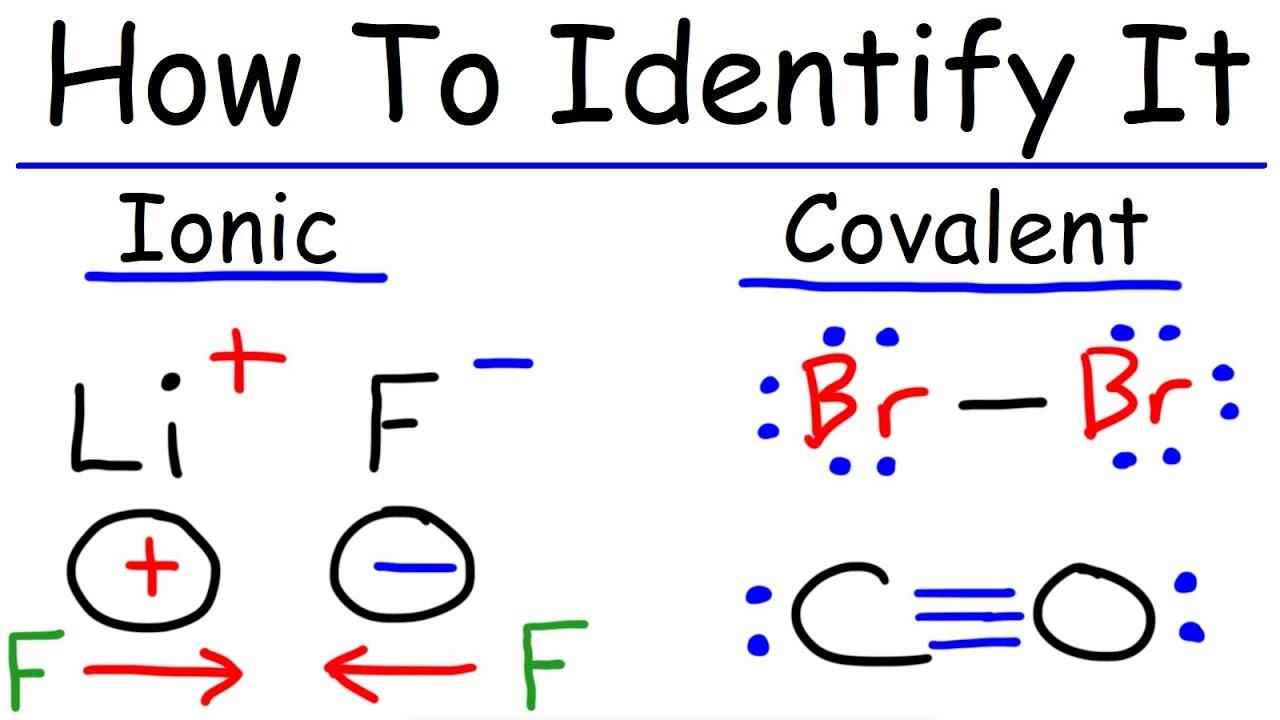
What S The Difference Between An Ionic Bond And A Covalent Bond Quora
Comments
Post a Comment